manganese electronic configuration|Manganese Electron Configuration (Mn) with Orbital : Clark The electron configuration of manganese shows that the last shell of manganese has two electrons and the d-orbital has a total of . Tingnan ang higit pa For STL we have found 385 definitions.; What does STL mean? We know 385 definitions for STL abbreviation or acronym in 8 categories. Possible STL meaning as an acronym, abbreviation, shorthand or slang term vary from category to category. Please look for them carefully. STL Stands For: All acronyms (385) Airports & Locations (6) Business & .7 talking about this
PH0 · What is the electron configuration of manganese?
PH1 · Manganese Orbital Diagram: Understanding the Electron
PH2 · Manganese Ground State Electron Configuration
PH3 · Manganese Electron Configuration (Mn) with Orbital
PH4 · Manganese (Mn)
PH5 · Manganese
PH6 · Electron Configuration for Mn, Mn2+, Mn3+ , and Mn4+ (Manganese and
PH7 · Electron Configuration for Mn, Mn2+, Mn3+ , and Mn4
PH8 · Electron Configuration for Manganese (Mn, Mn2+, Mn4+)
PH9 · Electron Configuration for Manganese (Mn, Mn2+, Mn4+)
PH10 · Chemistry of Manganese
SarsBoy666,free videos, latest updates and direct chatThe verification of ratings for the January 2024 Architect Licensure Exam (ALE) will be available online few working days after the posting of results. Successful takers can refer to PRC Official site and use the Verification Page to know their board examination passing rate.
manganese electronic configuration*******The total number of electrons in manganeseis twenty-five. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in manganese in specific rules in different orbits and orbitals is called the electron configurationof manganese. The electron configuration . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit pa
Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit paManganese Electron Configuration (Mn) with Orbital The electron configuration of manganese shows that the last shell of manganese has two electrons and the d-orbital has a total of . Tingnan ang higit pa
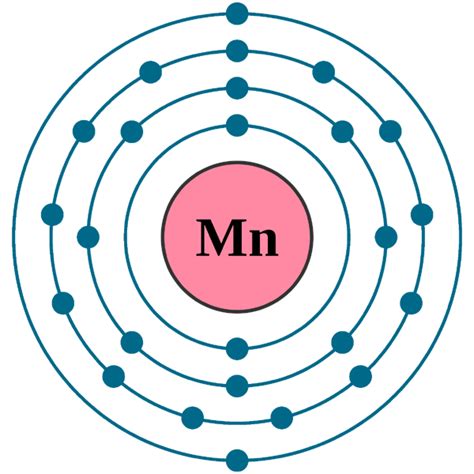
Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground-state electron configuration of manganese is 1s2 2s2 2p6 3s2 3p6 3d5 4s2. In the manganese ground-state electron configuration, . Tingnan ang higit pa To write the configuration for the Manganese ions, first we need to write the electron configuration for just Manganese (Mn). We first need to find the number of electrons for the Mn atom. Because of its valence electron configuration, it allows us to use it in different and unique ways that typically other elements cannot be used in. In biological systems .
Learn the electron configuration of manganese (Mn) and its main properties, such as oxidation states, applications and biological role. Find out how manganese is used in steel, batteries, . Electron configuration of Manganese is [Ar] 3d5 4s2. Possible oxidation states are +2,3,4,7. Electron Configuration. The periodic table is a tabular display of the chemical .
A fungal enzyme, manganese peroxidase, oxidizes manganese +2 atoms to manganese +3, which are then sent to the tiny spaces within the wood lattice. Manganese +3 is highly reactive .Learn about the chemical and physical properties, states, energy, electrons, oxidation and more of manganese (Mn), a transition metal with atomic number 25. See its electron configuration, .
Learn about the Manganese electron configuration here and make the systematic learning of the chemical element. The article provides thorough knowledge on the electron configuration of the Manganese so that .Learn how to visualize the electron configuration of manganese (Mn) using an orbital diagram. See the distribution of electrons in the 1s, 2s, 2p, 3s, 3p, and 4s orbitals, and the magnetic .
Let's find the ground state electron configuration of Manganese! A single Manganese atom has 25 protons and 25 electrons, but how do we know where Manganese .
To write the configuration for the Manganese ions, first we need to write the electron configuration for just Manganese (Mn). We first need to find the numb.Manganese (Mn) has an atomic mass of 25. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. ChemicalAid. . Electron Configuration [Ar] 4s 2 3d 5: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5: Orbital Diagram. Nuclear. Radioactive: No: Isotopes. Symbol Mass Number Relative Atomic Mass Isotopic Composition .Electron configuration of Manganese is [Ar] 3d5 4s2. Possible oxidation states are +2,3,4,7. The most common oxidation states of manganese are +2, +3, +4, +6, and +7, though all oxidation states from −3 to +7 have been observed. .
manganese electronic configurationLa configuration électronique du manganèse est 1s2 2s2 2p6 3s2 3p6 3d5 4s2. Le manganèse est l'élément chimique du tableau périodique des éléments situé dans le groupe 7, son symbole est Mn et son numéro atomique est 25. Today in this video, we will help you determine the electron configuration for the Manganese element. For determining its electron configuration, we first lo.Manganese. Full electron configuration of manganese: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 2 chromium ← manganese → iron. © 2009-2016 | www.prvky.com | kontaktkontakt

Filling Transition Metal Orbitals. The electron configuration for the first row transition metals consists of 4s and 3d subshells with an argon (noble gas) core. This only applies to the first row transition metals, adjustments will be necessary when writing the electron configuration for the other rows of transition metals. The noble gas before the first row of . Due to manganese's flexibility in accepting many oxidation states, it becomes a good example to describe general trends and concepts behind electron configurations. Figure \(\PageIndex{2}\): (left) A rough fragment of lustrous silvery metal (CC BY-SA 3.0; Tomihahndorf via Wikipedia ) (right) Some of the Lascaux cave paintings use manganese .The ground state electron configuration of ground state gaseous neutral manganese is [Ar].3d 5.4s 2 and the term symbol is 6 S 5/2. Schematic electronic configuration of manganese. The Kossel shell structure of manganese. The electron configuration of manganese, atomic number 25, is #"1s"^2"2"^2"2p"^6"3s"^2"3p"^6"3d"^5"4s"^2"#. The diagram below represents the electron configuration as an orbital diagram. Answer link. Related questions. How do electron configurations in the same group compare?Electronic configuration of the Manganese atom in ascending order of the levels: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 2 Reduced electronic configuration Mn: [Ar] 3d 5 4s 2. Below is the electronic diagram of the Manganese atom Distribution of electrons over energy levels in the Mn atom 1-st level (K): 2
Manganese, on the other hand, has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5 and a noble gas configuration of [Ar]4s 2 3d 5, resulting in one unpaired electron in each 3d sub-orbital. Similar to copper, the exchange energy is maximized since the 3d sub-orbital is exactly half-filled with 5 electrons without the need to .
manganese electronic configuration Manganese Electron Configuration (Mn) with Orbital Electron configuration: [Ar] 3d 5 4s 2: Density @ 20 o C: 7.43 g/cm 3: Show more, including: Heats, Energies, Oxidation, Reactions, Compounds, Radii, Conductivities. Atomic volume: . Manganese compounds have been used . Due to manganese's flexibility in accepting many oxidation states, it becomes a good example to describe general trends and concepts behind electron configurations. Figure \(\PageIndex{2}\): (left) A rough fragment of lustrous silvery metal (CC BY-SA 3.0; Tomihahndorf via Wikipedia ) (right) Some of the Lascaux cave paintings use manganese . Let's find the ground state electron configuration of Manganese! A single Manganese atom has 25 protons and 25 electrons, but how do we know where Manganese . Chemistry Electron Configuration Electron Configuration. 1 Answer Kris Caceres Jan 15, 2016 #[Ar] 4s^2 3d^5# Explanation: Mn has an atomic number of 25. Simply use this information to obtain its electronic configuration. Mn: #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^5# or simply #[Ar] 4s^2 3d^5#. Answer link. Related questions .
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum number of the .Manganese: Manganese is a d-block element having an atomic number 25 and atomic symbol Mn. It belongs to Group-7 and the fourth period. It is a hard silvery transition metal. Electronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration.
Planet Football is your destination for fun and challenging football quizzes, games and trivia. Test your skills and knowledge of the beautiful game.A PCIe is a high-speed serial computer expansion bus standard that connects devices such as graphics cards, solid-state drives, and network adapters to a computer’s motherboard. PCIe is a newer .
manganese electronic configuration|Manganese Electron Configuration (Mn) with Orbital